As the health care community prepares for flu season, one key challenge is determining whether patients have COVID-19 or seasonal influenza, given that both have similar symptoms. A laboratory test is needed to accurately diagnose either illness. This increased need for testing has the potential to tax a healthcare system that’s already severely resource-constrained.
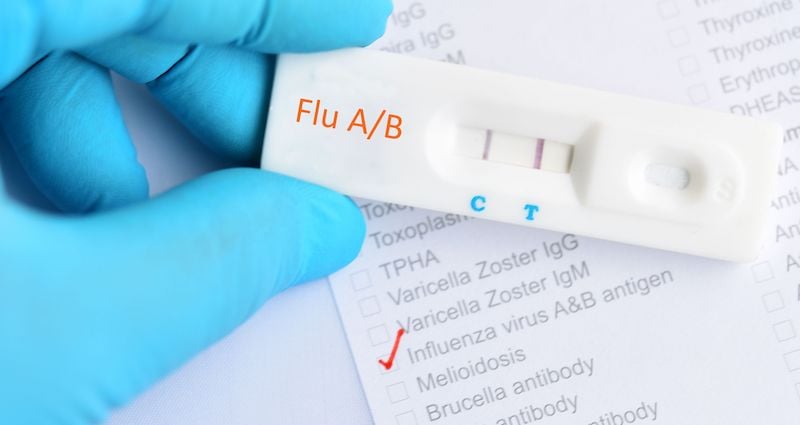
What is the combined nasal test for flu and COVID-19?
A combined nasal test for the flu and for COVID-19 is a single swab test designed to look for both the flu virus and COVID-19 virus. Health care providers now have the option of providing a combined nasal test to detect the presence of COVID-19 and influenza A and B.
This multiplex test is performed the same as the COVID-19 swab test. Due to the similarity of flu and coronavirus symptoms, the multiplex test provides much needed rapid direction as to how to treat a patient’s medical needs.
Dual flu and COVID-19 tests have been approved
Fortunately, several multiplex tests that can detect both flu and COVID-19 have been issued emergency use authorizations by the FDA. The CDC Influenza SARS-CoV-2 Multiplex Assay is a real-time reverse-transcription polymerase chain reaction (RT-PCR) process that can detect and differentiate RNA from SARS-CoV-2, influenza A virus and influenza B virus in upper and lower respiratory specimens.
Several similar tests have also received FDA authorization, including Qiagen’s QIAstat-Dx, Roche’s cobas®, BioMerieux’s BioFire and a dual test from LabCorp that can also diagnose respiratory syncytial virus (RSV).
Flu test versus COVID 19 test
The biggest benefit of the multiplex test is that through rapid diagnosis there is no delay in providing appropriate treatment.
Common signs associated with both illnesses include fever, cough and fatigue. Patients with either virus may also report less-common symptoms such as muscle aches, gastrointestinal upset, congestion, sore throat, runny nose, loss of smell and headache.
While testing is the only way to rule in or rule out influenza virus or SARS-CoV-2, this study found that the order of symptom occurrence may provide insight into a potential diagnosis. Early indicators can help identify which patients to test quickly and provide useful guidance around taking precautions such as quarantining if COVID-19 is suspected. This is particularly important given that COVID-19 is two to three times more contagious than influenza.
Given the overlap in symptoms between influenza and COVID-19, this is critical. While symptoms can range in severity for both COVID-19 and flu, the overlap of symptoms can make it difficult to diagnose based on symptoms alone.
There’s a lot of overlap between flu symptoms and those associated with COVID-19. A 2020 study provides a few clues that may help with diagnosis. But the only way to know for certain is to test.
Researchers at the University of Southern California modeled and analyzed data from thousands of patients worldwide who were diagnosed with COVID-19, influenza, SARS (severe acute respiratory syndrome) and MERS (Middle Eastern respiratory syndrome). Their analysis showed that a cough is most likely the first symptom of the flu. In contrast, a fever is most often the first symptom of COVID-19 as well as SARS and MERS.
|
Symptom |
Flu |
COVID-19 |
|
Fever or chills |
Yes; often lasts 3-4 days |
Yes |
|
Cough |
Yes |
Yes |
|
Shortness of breath or difficulty breathing |
Yes |
Yes |
|
Fatigue |
Yes |
Yes |
|
Sore throat |
Sometimes |
Yes |
|
Runny or stuffy nose |
Sometimes |
Yes |
|
Muscle pain or body aches |
Yes; often severe |
Yes |
|
Headache |
Yes |
Yes |
|
Vomiting and diarrhea |
Sometimes; more common in children |
Yes |
|
Change in or loss of taste or smell |
Sometimes |
Frequent |
Source: CDC
Another interesting observation was that the upper GI tract tended to be affected before the lower GI tract in COVID-19 cases; the inverse was true for MERS and SARS cases. A very small percentage of COVID-19 patients experienced diarrhea as an initial symptom. However, each of these patients went on to eventually have pneumonia or respiratory failure, which researchers hypothesize meant they had a more aggressive form of COVID-19. This could also be helpful information for clinicians to keep in mind, though more research is needed to support this theory.
And new variants of both COVID-19 and the flu may affect these symptoms,
What are the benefits of the dual COVID and flu test?
Normally two swabs would be needed to detect COVID-19 and influenza, one swab for each type of test. A multiplex test, however, such as the CDC Influenza SARS-Co-V (Flu SC2) Multiplex Assay can be done with one swab for the benefit of patients and healthcare professionals.
- A dual test is more comfortable for patients: Taking one sample is faster for patients and also means less discomfort. Worried patients can also get comprehensive results more quickly instead of waiting for two test results.
- A dual test drives faster diagnosis: When a medical professional can collect more information with a single test, they can more rapidly confirm diagnosis and make informed and targeted treatment decisions.
- A dual test frees up laboratory resources: When a single COVID and test can provide insight into multiple ailments, laboratories are able to conserve the use of reagents and other testing materials, while processing more tests in a given time period.
- A dual test helps control virus spread: Public health laboratories can use this dual test to collect data necessary for ongoing influenza surveillance while testing for COVID. Data from testing can help public health officials control the spread of both influenza and COVID-19.
What healthcare providers need to know for 2022-2023 season
Indicators point to a severe 2022-2023 flu season, making it important to prepare your office for a swell in influenza-like illness testing, in addition to the potential for COVID testing. The FDA notes that it remains possible for an individual to be infected simultaneously with influenza A virus, influenza B virus, and/or SARS-CoV-2, making it particularly critical to have dual tests on hand.
The CDC, in operation with other government agencies, makes recommendations for best use of existing vaccines and supports the development of new and better vaccines. Overall, the CDC is encouraging anyone over the age of 6 months to get their flu vaccination by the end of October 2022, but acknowledges that vaccination at any time during the peak of flu season can provide critical protection. CDC also notes that it is acceptable to get a flu vaccine at the same time as a COVID-19 vaccine, although the organization encourages getting up to date on vaccinations without delay if one or the other is unavailable at a given time.
Puritan swabs can be used for combined flu and covid testing
As one of only two suppliers worldwide who produce the specialized swabs CDC recommends for nasopharyngeal sample collection, Puritan Medical has been at the epicenter of COVID-19 testing. Solutions like our HydraFlock and PurFlock sterile ultrafine flock swabs afford health care providers added certainty in diagnosing patients presenting with these common symptoms by providing better cell yield in the specimen compared to alternative solutions.
As COVID-19 testing in particular continues to evolve, Puritan continues to update its testing resources to connect healthcare providers with the best solutions for patient health and safety.
With the 2022-2023 flu season underway, combined flu and COVID-19 testing may prove critical in relieving some of the pressure and provide quick, accurate information to patients, practitioners and the community at large.
Looking for more? Check out these printable PDFs on how to collect a nasal and nasopharyngeal specimen.
Your success story starts here - Puritan Medical Products empowers you to transform concepts into reality. Launch your journey to excellence with personalized custom swab kits.
DISCLAIMER: The information on this site is not intended or implied to be a substitute for professional medical advice, diagnosis, or treatment. All content, including text, graphics, images, and information, contained on or available through this web site is for general information purposes only. Please consult with a licensed medical professional regarding the application of products.