 Whether your lab utilizes an automated platform for specimen analysis or traditional methods to culture specimens, a recent study confirms you can be sure of reliable results with Puritan’s Opti-Swabs®.
Whether your lab utilizes an automated platform for specimen analysis or traditional methods to culture specimens, a recent study confirms you can be sure of reliable results with Puritan’s Opti-Swabs®.
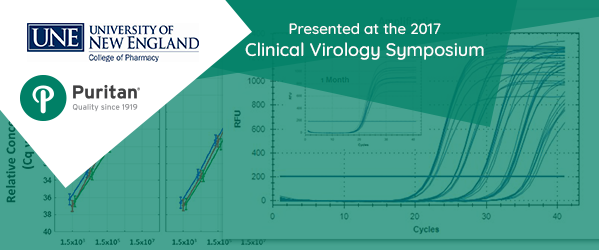
The study, presented at the 2017 Clinical Virology Symposium in Savannah, Georgia, was undertaken to confirm the hypothesis that genetic material could be successfully stored and transported for up to one week without any negative effect. Researchers shared results that show both bacterial and viral pathogens were successfully detected and accurately quantified by Q-PCR.
Puritan Medical Products Company, LLC, formulates an expanding line of transport media devices in its state-of-the-art lab in Guilford, Maine. Opti-Swab was the second transport media device Puritan offered, launched in 2012. This liquid Amies medium has long been accepted for the transport of bacterial specimens to the lab for processing.
Today, technology is relying more heavily on the detection of the DNA/RNA of a microorganism, and microbiologists must now gear their work to the genetic material of specimens. They will rely on identification of the pathogen by detecting it’s DNA. Once very time consuming and costly, PCR is now so efficient it saves both time and money. But, the technology requires a specimen with genetic integrity. Will this same product serve for this purpose?
This takes us to the study conducted at the University of New England. Samples of Haemophilus influenza and Neisseria gonorrhoeae were incubated and influenza A virus propagated to prepare appropriate dilutions for the study. The dilutions were absorbed by sterile swabs that were then placed in vials containing Puritan Opti-Swab medium and held for 24, 48, and 168 hours at either room temperature (RT) or 4° C. DNA/RNA was isolated and quantitative polymerase chain reaction (Q-PCR) detected the target pathogens.
It was found that DNA and RNA yields from all samples were sufficient for multiple PCR assays, regardless of storage temperature or time.
Methods described in the study tell us commercially available tools were used, including Promega and Invitrogen purification kits, the Qiagen virotype influenza A qRT-PSR assay, and the BioRad CFX96™ PCR Detection System.
What does this mean for the lab tech? It means that the genomic material of both bacterial and viral specimens can be transported successfully using Puritan’s Opti-Swab, whether they are to be analyzed immediately or as much as one week later, and whether stored under refrigeration or at room temperature.
The bottom line: Opti-Swab is a reliable medium for the collection and transport of nucleic acids and appropriate for collection and transport of specimens for later qPCR analysis.
As noted in the study:
“Storage in Puritan Opti-Swab® medium does not have a negative effect on the ability to detect and accurately quantify samples, whether bacterial pathogens, H. influenza and N. Gonorrhoeae, or the viral pathogen Influenza A. This data further supports the efficacy of this medium in collection and transport of nucleic acids from clinical specimens prior to qPCR assays.”
You can view the poster here on our site for more details about the study. For more information about Opti-Swab, contact a knowledgeable sales representative today.