When you’ve been in business for over 100 years, you get used to change, and here at Puritan, our 102-year-old company has seen it all – from toothpicks to pandemics.
With each change, we grow as a business, and I believe we get better. Global regulations and standards continue to evolve with the changes in technologies, global harmonization, and changing user, patient, and community needs, and Puritan has been at the forefront of those changes.
What is the MDR?
In 2017, the European Union adopted new regulations for medical devices to ensure better protection of public health and patient safety. The new regulations, called the MDR, set higher standards of quality and safety for medical devices, like the ones Puritan makes here in Maine, including our swabs.
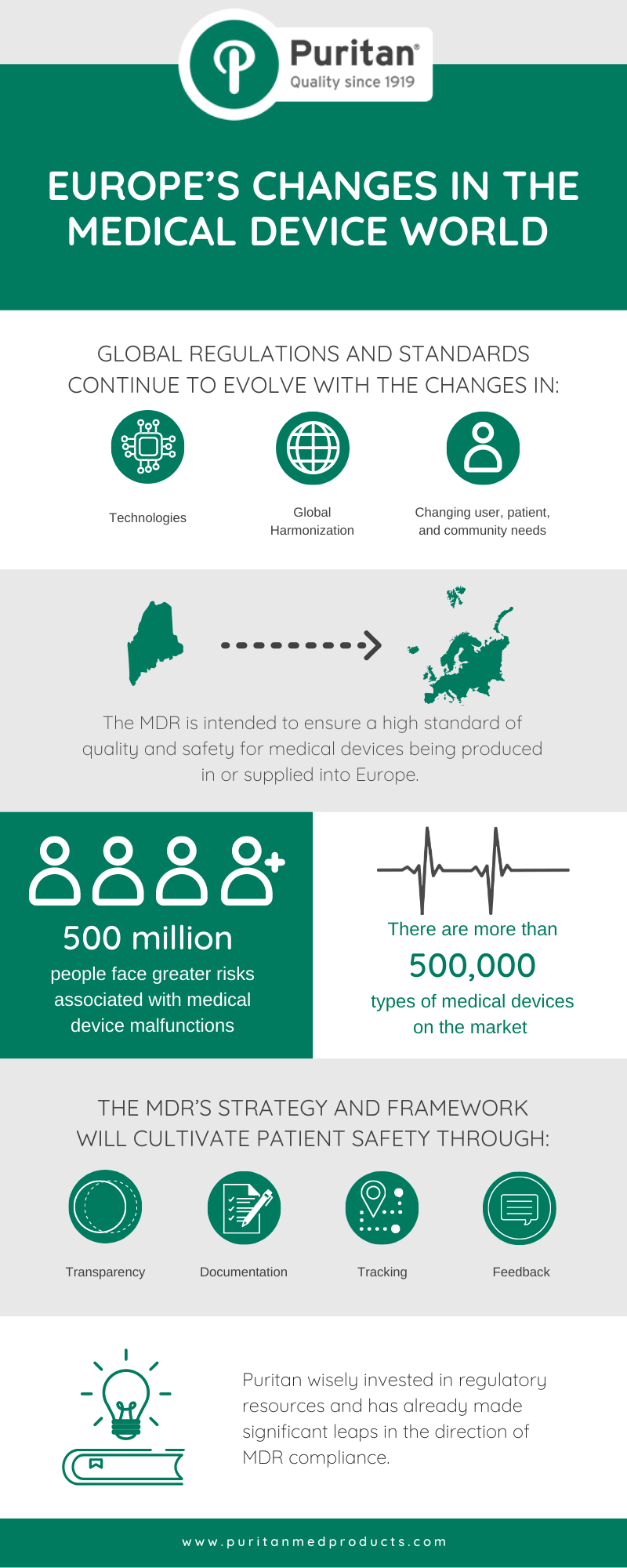
The MDR is intended to ensure a high standard of quality and safety for medical devices being produced in or supplied into Europe and it went into effect this May.
How the MDR Affects the Medical Device Industry
Unless you work in the medical device industry, the new regulations would have gone unnoticed. In our industry though, it’s a big deal. These regulations have raised the responsibilities of medical device companies selling in many European countries where an aging population of 500 million people face greater risks associated with medical device malfunctions.
As one of just a couple medical swab producers that sell within the EU, Puritan’s current Quality System and CE Marked medical devices already met the EU’s former regulatory standards, the Medical Device Directive (MDD), and are considered “legacy devices” and will remain valid until the expiry date of our EC Certificate, which is in 2023.
That means we have until then to comply with the MDR and I am confident we will meet the new regulations well in advance of this deadline.
How Puritan Will Meet MDR Changes
I am proud that Puritan is stepping up to meet the challenges of the new regulations. There are more than 500,000 types of medical devices on the market and most people will need to use a medical device at some point in their lives which is why we take safety regulations very seriously. In fact, Puritan regularly goes beyond our own quality control satisfaction because it is extremely important to us that every product we make is the best – and safest – of its kind.
The MDR’s strategy and framework will cultivate patient safety through transparency, documentation, tracking, and continuous feedback loops. Over time, Puritan will gain valuable production and post-market data that will feed back into the Risk Management and Improvement aspects of Puritan’s Quality System.
Manufacturers of medical devices have the main role in complying with the EU’s MDR and has by far the largest number of obligations to fulfill. Puritan is ready to fulfill these requirements.
The MDR implementation is a significant project for all device manufacturers (small and large) that must be addressed for continued access to the EU market. Puritan wisely invested in Regulatory resources and has already made significant leaps in the direction of MDR compliance.
Puritan is a family-run business, and I am proud, as the third generation at the helm, to have helped to guide us from our former focus in the wood products industry to a medical products company that offers value around the globe. Whenever there are big changes, I believe that provides us all with an opportunity to rise and meet the challenge and hopefully learn something and improve along the way.