Every September, just about everyone gets sick. Back-to-school fever—brought on by all that mixing and mingling—hits kids, parents, and seemingly everyone else.
Doctors’ offices and clinics find themselves inundated with RIDT flu tests, while long-term care facilities find themselves performing viral cell cultures and RT-PCR tests on a regular basis.
But how do facilities determine which tests are appropriate for which cases? And what’s the difference between the popular methods of flu testing? Below we look at the three most common flu testing methods, their applications, and the various flu testing tools healthcare facilities should have on hand.
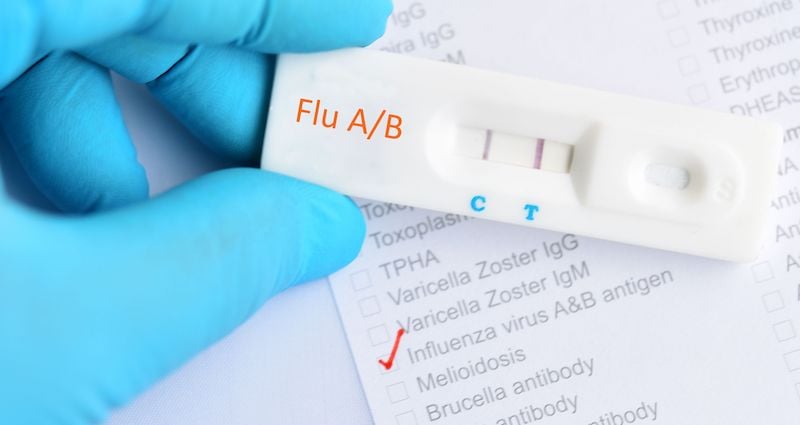
Rapic Diagnostic Influenza Test (RIDT)
RIDTs are the most common testing method used to diagnose the flu because of their near-instantaneous results and ease of appropriate specimen collection. These tests, which usually rely on nasopharyngeal samples, can be completed in a doctor’s office, pharmacy, clinic, nursing home, and other healthcare institution. They are useful in diagnosing illness during periods of acute respiratory disease and helping to establish the prevalence of influenza in a specific population.
However, not all RIDTs can distinguish between influenza A and B; and no RIDTs can distinguish between the varying strains of influenza A. Additionally, when compared with viral cell cultures and RT-PCR or other molecular assay testing methods, RIDTs have the lowest sensitivities and the highest rate of false-negatives, meaning they only detect up to 70% of flu cases.
Viral Cell Culture
Viral cell culture is the oldest and most reliable method of diagnosing influenza, but it also takes the longest—with results available between 3 and 10 days after sampling. That’s because viral cell cultures require collected specimen to be sent to a lab where it is then grown in culture over a period of several days and monitored for cellular response to viral infection.
For this reason, viral cell cultures are most appropriate for documenting which strains of influenza are active in a given population or geographical region and documenting which antiviral agents are effective treatments. They are also sometimes used to confirm or refute a rapid influenza test result.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR is a relatively new type of molecular assay that uses isothermal amplification of viral cells. While RT-PCR is more sensitive and specific for detecting influenza than the other testing options, it is also generally more expensive.
The most common use cases for RT-PCR tests is the identification of the influenza virus in populations suffering from acute respiratory outbreaks. Popular examples include hospitals, elder-care facilities, long-term care facilities, and the like.
The table below breaks down these three methods by the testing timeline, acceptable specimen types, potential specimen collection tools, common use cases, and test accuracy.
How the Most Popular Flu Tests Compare
| Testing Method | Test Results Timeline | Specimen Types | Specimen Collection Tools | Use Cases | Accuracy |
|---|---|---|---|---|---|
|
RIDT |
15-30 minutes | NP swab, throat swab, nasal wash with suction, nasal aspirate | Flocked np swab, viral transport media, bulb or syringe-style aspiration kit, isotonic saline & suction pump with trap | Completed in doctor's office, differentiate influenza from other infections, determine whether antiviral drugs are appropriate | High rate of false-negative results |
| Viral Cell Culture (serology) |
3-10 days | NP swab, throat swab, NP or bronchial wash, nasal or endotracheal aspirate, sputum | Flocked np swabs, flocked throat swabs, viral transport media, bulb or syringe-style aspiration kit, suction pump with trap, sputum container | Confirmation of a positive rapid test result, confirmation of a negative test result, documenting which strains of influenza are active, documenting which antiviral agents are effective treatments | Highest viral specificity |
| RT-PCR (molecular assay) |
2-8 hours | NP swab, throat swab, NP or bronchial wash, nasal or endotracheal aspirate, sputum | Flocked np swabs, flocked throat swabs, viral transport media, bulb or syringe-style aspiration kit, suction pump with trap, sputum container | Diagnose influenza A (especially in seriously ill/hospitalized patients), help track influenza outbreaks | Most sensitive and specific for detecting influenza |
Notice any commonalities? Due to their ability to collect and elute more specimen than other swab types, flocked swabs are the preferred choice for nasopharyngeal and throat swab tests.
A solid stash of flocked swabs is the bare minimum your facility should have on hand for testing and diagnosing the flu. You'll also want to stock up on a reputable viral transport medium.
Want more information about swabbing for the flu? Click below to link to our new, comprehensive Puritan flu portal.
Conclusion
For more information on flu testing products, please contact us today.
Sources: CDC, AACC, University of Colorado Health, Stanford School of Medicine